French attractiveness and competitiveness : Leem survey of the health industry
Two major factors for success: greater emphasis on innovative technology and renewed partnerships
Is France an attractive investment option for the international managers of pharmaceutical companies? On what basis? What concrete measures will enable France to reindustrialise and remain a major country in pharmaceuticals over the next ten years?
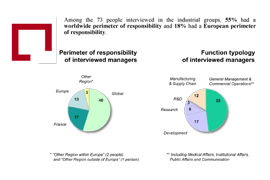
To provide objective answers to these questions, particularly in the framework of the CSIS (Strategic Council for Healthcare Industries), in 2009 the French Pharmaceutical Companies Association (Leem) commissioned AEC Partners to conduct a study, the first in France, amongst major pharmaceutical groups. This qualitative study was based on interviews with 73 international managers of pharmaceutical companies on several continents to find out their views of France as an investment destination. The interviewees represented nineteen major pharmaceutical companies accounting for over two thirds of the French market.
The qualitative results presented today paint an encouraging picture and reveal the challenges to be met:
First lesson of the study: France remains a relatively attractive and legible market undergoing restructuring
- By size, it is one of two large European markets and the fourth global market behind the United States, Japan and Germany;
- As with other Western countries, however, it is trapped between two very attractive geo-economic forces: the United States and emerging countries.
Second lesson: an excellent industrial tradition, yet whose social environment prompts mixed reactions and which must place greater emphasis on innovative technology
- The position of leading country for the production and export of pharmaceuticals in Europe, if not the world, can be explained by the French industrial environment: quality of the engineers and technicians, transport and telecommunications infrastructure, strong industrial tradition in the pharmaceutical sector and quality of the pharmaceutical distribution system;
- However, the social environment is viewed much less positively due to two main factors: organisation of working time and labor relations, particularly in public transport and the civil service,
- Yet this view is nuanced by industrialists who have a more intimate knowledge of France, who point out the difference between perception and reality. In particular, they stress the high productivity of the French workforce and the reforms undertaken, and note that other countries also have a complex and restrictive working environment.
Third lesson: a highly competitive academic R&D environment, with underexploited potential, where cooperation and partnerships are required
- France has significant strengths to be a competitive global player in R&D: strike force of public research in the biomedical sector, excellence in the areas of engineering, mathematics, physics, quality of the healthcare system and skilled clinicians, opinion leaders with international reputations in several therapeutic areas;
- Yet in the eyes of international managers, France, unlike other countries, is unable to turn its strengths into competitive advantages through lack of a strong investment policy for life science research, the fragmentation of public research, the relative dispersion of public investment and the shortage of public-private partnerships.
Fourth lesson: the positive perception of a political environment that has incorporated dialogue and consultation
- France stands apart from the rest of Europe with a clear political will to consider healthcare industries as a strategic sector, with a range of concrete measures and initiatives over the last three years: CSIS, R&D Dating meetings, General State of Industry meetings, ”Great Loan”, implementation of AVIESAN (French National Alliance for Life and Health Sciences) in 2009 and reform of the Research Tax Credit in 2008. These structural developments are viewed positively by economic decision makers.
The healthcare industry, a strategic sector
“Attractiveness isn’t divisible,” stresses Christian Lajoux, Chairman of the Leem. “France is a major country in life sciences and pharmaceuticals. For years it’s been one of the leading European exporters of pharmaceuticals with a trade surplus of nearly €7 billion in 2009, making it the fourth contributor to the French balance of payments. The pharmaceutical industry is undergoing a radical transformation: its products, research approaches, the organisation of companies and geography. To ensure that the transformation is successful, France must turn its strengths into competitive advantages and above all maintain the legibility and visibility of market regulation. The government has understood this; the industry must, for its part, meet its commitments and enable the sector to strengthen its strategic nature in the same way as energy, transport or space industries. I’m convinced that we have the duty to maintain our international standing and have it bear fruit.”
The study authors emphasise three necessary conditions to improve the effectiveness of research in France’s healthcare sector:
- Close the gap between perception and reality in relation to the French environment to increase the attractiveness of our country;
- Open dialogue with all stakeholders in the French environment in order to develop a convergence of interests, consistent policies and united approaches;
- Continue the reforms reorganising research and implement a proactive and ambitious policy to promote French excellence and develop public-private partnerships.
What influences the investment decisions of international pharmaceutical companies?
The study conducted by AEC Partners on behalf of the Leem reveals the decisive factors for investment decisions by managers of pharmaceutical companies:
- Criteria linked to the size and growth potential of the market under consideration
- Criteria “internal” to companies: the capillarity of investments (a large part of investments are made in pre-existing sites); the quality of local management and past performances of the subsidiaries or sites; the nationality of the investing group: there is an inclination, particularly amongst the Americans and French, to invest in home countries.
- Criteria linked to the country’s environment. For R&D managers, the main criteria taken into account are the quality and accessibility of skills that can work within networks (development of partnerships with public research). For clinical research managers, the quality/speed/cost triangle takes precedence: quality and risk management of clinical development plans, speed of implementation of clinical plans and cost. In terms of industrial affairs, there is a trend towards reconversions of existing sites and positioning on productions with high added value. For bioproduction, the proximity of R&D centres is an important element. For commercial operations decision makers, finally, the predictability and recognition of innovation are major criteria. Pricing levels, the legibility of healthcare policy and the speed and degree of market penetration are particularly considered.

